Cancervax Announces Promising New Drug to Treat a Deadly Children’s Cancer
Company plans to launch FDA IND enabling studies needed to apply for approval to proceed with human trials
Santa Barbara, CA, April 16, 2024 (GLOBE NEWSWIRE) -- Cancervax, Inc., a pre-clinical biotechnology company creating a better way to treat cancer, the developer of breakthrough cancer drugs that will use the body's immune system to treat cancer, today announced that its UCLA research team has created a promising new drug candidate for treating recurrent Ewing sarcoma, a deadly children's cancer.
Ewing sarcoma is a rare and deadly bone and soft tissue cancer that primarily affects children and young adults. There are currently no treatment options for recurrent Ewing sarcoma, which has a near 100% death rate.
The research program, funded by Cancervax, is led by a world class team of cancer researchers and oncologists who run the Ewing Sarcoma Program at the UCLA Jonsson Comprehensive Cancer Center. The principal investigators include Dr. Satiro De Oliveira, Dr. Noah Federman, and Dr. Steven Jonas.
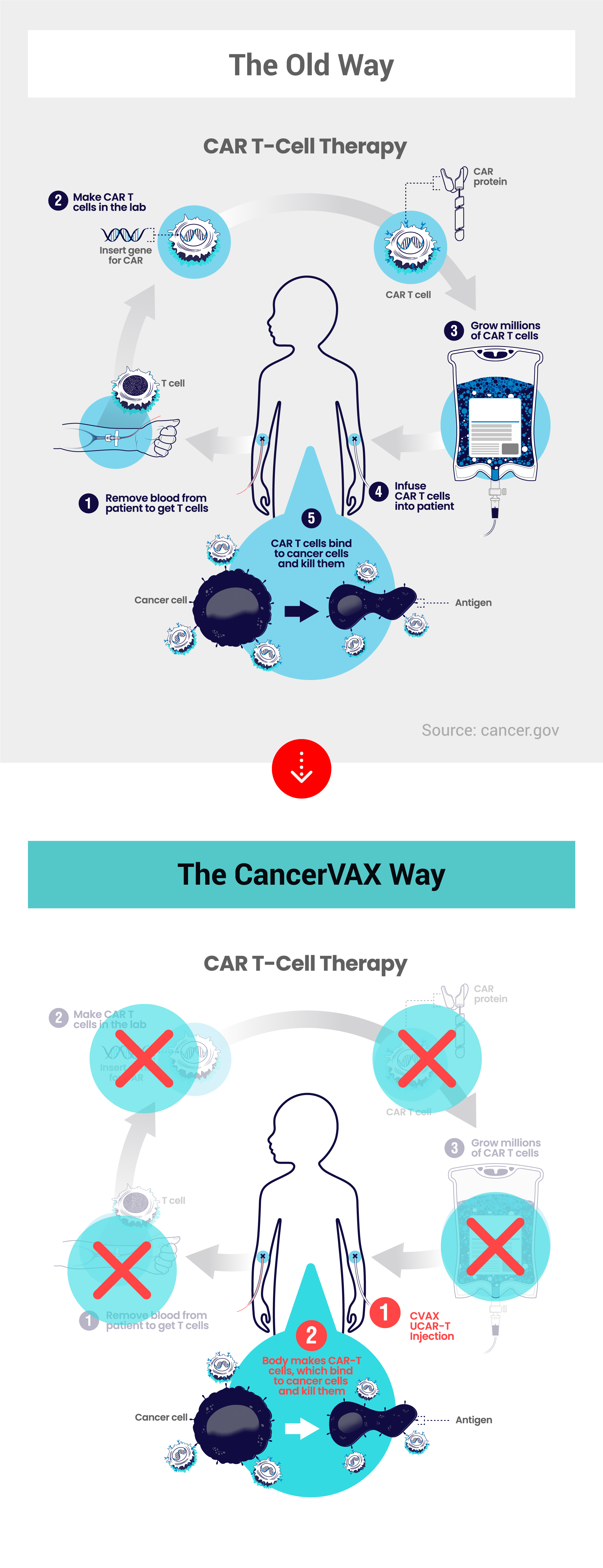
After nearly 3 years of ups and downs, which are typical to biotech development programs, the passion and commitment of Dr. De Oliveira and the other team members have resulted in a promising “bispecific antibody vaccine” created specifically to treat Ewing sarcoma. This treatment vaccine can target and attach to Ewing sarcoma cancer cells, and attract the body’s natural killer T-cells (immune system cells) to the tumor for destruction; hence the “bispecific” descriptor. In extensive laboratory cell studies, several candidates exhibited meaningful T-cell killing of Ewing sarcoma cells, which meant the vaccine was doing its job of bringing T-cells to the tumor cells for destruction.
Based on the positive data, Dr. Noah Federman, co-principal investigator and Director of the Pediatric Bone and Soft Tissue Sarcoma Program at UCLA, has recommended that Cancervax proceed with IND enabling studies for preparing an application to the FDA for human clinical trials. An IND (Investigational New Drug) application includes toxicity experiments, manufacturing information, and clinical protocols. More information about this process can be found on the FDA website: http://fda.gov/drugs/types-applications/investigational-new-drug-ind-application
Cancervax CEO, Byron Elton, said, "Cancer vaccines, also known as cancer immunotherapies, are the latest weapons in the war against cancer. This new type of cancer drug uses the body's own immune system to treat cancer. We believe this is a better way to treat cancer rather than the old methods of chemotherapy, radiation, and surgery. We have been in research mode for nearly 3 years and the creation of our first cancer drug candidate represents a major milestone which marks the turning point where we have become a viable biotech company. If we are successful with our Ewing sarcoma vaccine, we will help save many children from this deadly disease. If we are successful with our more comprehensive Universal Cancer Treatment project at UCLA, then we will help save people of all ages from many types of cancers."
About Us
Cancervax, Inc. is a pre-clinical biotechnology company developing breakthrough cancer drugs that will use the body's immune system to treat cancer. Working with a team of world class cancer researchers and oncologists at UCLA, we intend to create a Universal Cancer Treatment that will detect, mark, and destroy only the diseased cells exclusively. By inducing cancer cells to express a distinct marker absent in healthy cells, custom antibody drugs and the body's immune cells can precisely target and eradicate cancer cells. Also working with UCLA, we have created our first cancer drug candidate – a single-disease specific immunotherapy targeting Ewing sarcoma, a rare but deadly bone and soft tissue cancer primarily affecting children and young adults. Based on the positive data, we plan to launch FDA IND enabling studies needed to apply for approval to proceed with human trials. We look forward to the day when treating cancer will be as simple as getting a flu shot – a better way to treat cancer. To learn more, please visit www.cancervax.com
Forward-Looking Statements
This press release may contain “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations, and assumptions regarding the future of our business, plans and strategies, projections, anticipated events and trends, the economy, and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict, many of which are outside our control. Our actual results and financial condition may differ materially from those in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Any forward-looking statement made by us in this release is based only on information currently available to us and speaks only as of the date it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments, or otherwise.
Press Contact:
Cancervax, Inc.
Tel: (805) 356-1810
communications@cancervax.com