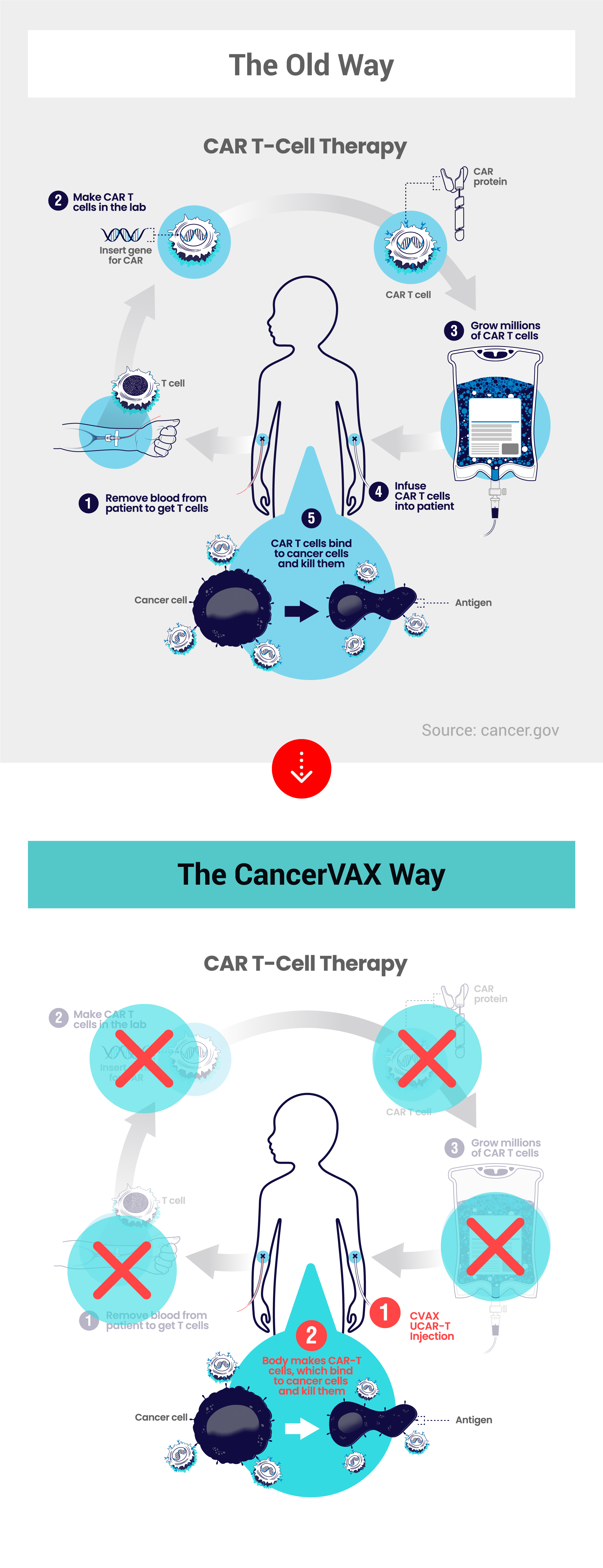
The CancerVax Way
- 10 million people died last year because the body does not easily recognize and kill cancer cells.
- However, the body is very good at killing diseases that it recognizes, such as measles.
- Our unique technology disguises cancer cells to look like measles and “tricks” the body into killing them.
Short Explainer Video
The CancerVax Way
- 10 million people died last year because the body does not easily recognize and kill cancer cells.
- However, the body is very good at killing diseases that it recognizes, such as measles.
- Our unique technology disguises cancer cells to look like measles and “tricks” the body into killing them.